Authors
- Abolfazl Abbaszadeh 1
- Akram Tehmasebi-Foolad 2
- Asghar Rajabzadeh 3
- Nasim Beigi-Brojeni 4
- Leila Zarei 3
1 Department of Surgery, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
2 Student research committee, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
3 Department of Anatomical Sciences, Faculty of Medicine, Lorestan University of Medical Sciences, Kharamabad, Iran
4 Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran
Abstract
Objective: The present study was aimed at assessment of effect of application of Chitosan/Nano Selenium biofilm on infected wound healing in ratsMethods: Sixty-eight male Wistar rats were randomized into four groups of 17 animals each. In group I (Normal) the wounds were created with no infection. In group II (MRSA), the wounds were infected with methicillin resistant Staphylococcus aureus (MRSA). In group III (MRSA/CHIT), animals with infected wounds were dressed with chitosan biofilm only. In group IV (MRSA/CHIT/NS), animals with infected wounds were dressed with Chitosan/Nano Selenium biofilm.Results: There were significant differences in comparisons of group IV and other groups, particularly in terms of cellular infiltration and neovascularization. During the study period, scores for neovascularization was significantly higher in group IV rats than other groups (P<0.05). Polymorphonuclear (PMN) and mononuclear (MNC) cell count and fibroblast cell proliferation in group IV were significantly higher than those of other experimental groups (P<0.05)Conclusion: Chitosan/Nano Selenium biofilm resulted in significant improvement in histopathological indices in full thickness infected wound healing.
Keywords
Introduction
Open wounds are particularly prone to infection, especially by bacteria, and also provide an entry point for systemic infections. Infected wounds heal less rapidly and also often result in the formation of unpleasant exudates and toxins that will be produced with concomitant killing of regenerating cells. Consequently, there is a need to stimulate healing and restore the normal functions of the affected part of the body to ease the discomfort and pain associated with wounds, preventing infection, and activating tissue repair processes [1]. Staphylococcus aureus (S. aureus) is an important cause of nosocomial infections in most health centers [2].
Methicillin-resistant Staphylococcus aureus (MRSA) is the most widespread bacterial pathogen causing various infections ranging from skin and soft tissue infections to serious invasive infections, such as pneumonia, endocarditis, bacteremia and sepsis [3,4]. It is estimated that Multi-drug resistant Staphylococcus aureus infections leads to high mortality with an associated annual health care costs [5,6]. Despite this high mortality rate, there are relatively few new antibacterial agents in the pharmaceutical pipeline [7]. Instead, the majority of antibiotics developed in the last decade are molecules re-engineered from existing antibiotic classes for which underlying resistance mechanisms are already present [8]. Therefore, effective new therapeutic options for treatment of infections caused by multidrug resistant S. aureus are urgently needed.
Well-designed scaffold with a suitable porous structure can support cell migration and guide vascular infiltration, making it an ideal dermal substitute for wound regeneration. Several in vitro studies that focused on determining the optimal mean pore size of collagen-based scaffolds indicated that large pores (≥250 μm) favor cell attachment, proliferation and migration [9]. The basic strategy of engineered tissue regeneration is the construction of a biocompatible scaffold that, in combination with living cells and/or bioactive molecules, replaces, regenerates, or repairs damaged tissues. The scaffold should possess suitable properties, like biocompatibility, controlled porosity and permeability, and, additionally, support for cell attachment and proliferation. This artificial “dermal layer” needs to adhere to and integrate with the wound, which is not always successful for the current artificial dermal analogues available [10].
Chitosan is a high-molecular weight natural polymer. It is nonpoisonous; it can accelerate wound healing, reduce blood cholesterol levels, stimulate the immune response and can be biologically decomposed. It has a stronger antimicrobial property compared to chitin in avoiding fungi because it has an active group that will bind to microbes, so it can inhibit microbial growth. Chitosan has a good chemical reactivity because it has a number of hydroxyl (OH) and amine groups (NH2) attaching to its chain. One of its important characteristics is that it has a positive charge in acidic solution. The substance is a stronger antifungal factor compared to chitin. In addition, chitosan is polycationic, so it can be used as a clotting agent [11-13]. An increasing number of products emerging from the application of nanotechnology to the science of wound healing is currently under clinical investigation. The nanoscale strategies, both carrier, drug related and scaffold target the main phases of wound repair [14].
In recent years, nanoparticles have emerged as important platforms to treat skin wounds. Silver, gold, and copper nanoparticles, as well as titanium and zinc oxide nanoparticles, have shown potential therapeutic effects on wound healing. Due to their specific characteristics, nanoparticles such as nanocapsules, polymersomes, solid lipid nanoparticles, and polymeric nanocomplexes are ideal vehicles to improve the effect of drugs (antibiotics, growth factors, etc.) aimed at wound healing. On the other hand, if active excipients are added during the formulation, such as hyaluronate or chitosan, the nanomedicine could significantly improve its potential. In addition, the inclusion of nanoparticles in different pharmaceutical materials may enhance the beneficial effects of the formulations, and allow achieving a better dose control [15].
Selenium is one of the essential trace elements for humans. The bioavailability of Se is related to its different chemical species. Recently, elemental selenium nanoparticles are attracting more and more attention due to their excellent high biological activity and lower toxicity [16]. Elemental selenium nanoparticles in liquid phase can be used as the materials for medical purposes [17]. For these applications, it is important to have good stability of elemental selenium nanoparticles in liquid phase. One of the effective methods for stability of nanoparticles in liquid phase is to add modifiers. Others used the chitosan as modifiers for the fabrication of elemental selenium nanoparticles [18]. The objective of the present study was to assess effect of Chitosan/Nano Selenium biofilm on infected wound healing in rats
Materials and Methods
Animals
Sixty-eight adult healthy male Wistar rats weighting approximately 240g were used and housed in individual cages under room temperature (22 ±3°C), and humidity (60 ± 5%) with natural light/dark cycle, and had ad libitum access to standard pellet diet and water throughout the study.
Ethical Considerations
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health followed in works of others [15]. The research project has received the confirmation of the Institution Ethics Committee with the number of IR.LUMS.REC.1397.114.
Preparation of Chitosan/Nano Selenium biofilm
Water-soluble chitosan solution was prepared using a method described by others [19]. Briefly, Medium molecular weight crab shell chitosan was dissolved (~400 kDa, 85% deacetylated, Sigma- Aldrich St. Louis, MO, USA) into an aqueous solution (1% v/v) of glacial acetic acid (Merck, Germany) to a concentration of 2% (w/v) while stirring on a magnetic stirrer-hot plate. The solution was stirred with low heat (50˚C) for 3 hours. The resultant chitosan solution was filtered through Whatman filter paper after vacuum filtration to remove any un-dissolved particles. For the preparation of elemental selenium nanoparticle sol, 1.5 mL 0.227 mol/L Vc was mixed with 1.0 mL 2.40 mol/L acetum, then the appropriate amounts of 5.36 mmol/L Se(IV) solution was added into the mixtures, the mixed solution was diluted to 10 mL. For the preparation of selenium nanoparticle-chitosan solution, appropriate amounts of chitosan solution were mixed with 1.5 mL 0.227 mol/L Vc and 1.0 mL 2.40 mol/L acetum, respectively. The appropriate amounts of 5.36 mmol/L Se(IV) solution was added into the mixtures, then the mixed solution was all diluted to 10 mL. Philips diffractometer was used to obtain X-ray diffraction pattern. Field emission scanning electron microscopy FESEM studies (Philips ES 30 KWO) were used to determine morphology of the scaffold (Figure1).
Fig. 1. Micrograph of scanning electron microscope to evaluate (A) ultra-structure of porosity of chitosan scaffold and (B) morphology of Chitosan/Nano Selenium biofilm.

Design of study
Sixty-eight rats were randomized into four groups of 17 animals each. Nine animals in each group were served for histological, five for planimetric studies and three rats for microbiological assessments. In group I (Normal) the wounds were created with no infection. In group II (MRSA), the wounds were infected with MRSA. In group III (MRSA/CHIT), animals with infected wounds were dressed with chitosan biofilm only. In group IV (MRSA/CHIT/NS), animals with infected wounds were dressed with Chitosan/Nano Selenium biofilm.
Wound creation and infection procedures
Rats were anesthetized by an intraperitoneal injection of ketamine (70 mg/kg of b. w.) (Alfasan, Netherlands) and xylazine (5mg/kg of b.w.) (Alfasan, Netherlands), the hair on their back was shaved and the skin cleansed with 70% alcohol solution. Following shaving and aseptic preparation, a circular excision wound was made by cutting away a full thickness of 10 mm in diameter excision circle wounds extending through the panniculus carnosus using a 10 mm dermal biopsy with homemade sterile punch. Small gauze was placed over each wound and then inoculated with 5 × 107 CFU of Staphylococcus aureus ATCC 43300. The methicillin-resistant S. aureus ATCC 43300 strain was commercially available. The pocket was closed by means of 4-0 nylon sutures and this procedure resulted in a local abscess after 24 h. The rats were returned to individual cages and they were examined daily. After 24 h, the wounds were opened, the gauze removed for quantitative bacterial cultures and treatment started. In Normal group, 0.1 mL sterile saline 0.9% solution was added to the wounds with no infection. In groups with infected wounds, the wounds were infected with MRSA and only treated with 0.1 mL the sterile saline 0.9% solution. The rats were placed in their individual cages warmed by a heater and allowed to recover fully from anesthesia.
Microbiological assessments
Briefly, for total bacterial count on days 7 and 14 of treatment after wound creation the granulated tissues were excised aseptically. Then, 0.1 g of sample was crushed and homogenized in sterile mortar containing 10 ml of sterile saline. The homogenized sample was serially diluted in tube containing 9 ml of sterile saline to 10-5. The diluted samples were cultured on plate count agar (Merck KGaA, Darmstadt, Germany) superficially and duplicated. The cultured plates were incubated at 37 ºC for 24 to 48 hours. After incubation, all colonies were counted and results described as CFU/g of granulation tissue [20].
Planimetric studies
Wound-healing property was evaluated by wound contraction percentage and wound closure time. Photographs were taken immediately after wounding and on days 6, 9, 12, 15, 18 and 21 post-wounding by a digital camera while a ruler was placed near the wounds. The wound areas were analyzed by Measuring Tool of Adobe Acrobat 9 Pro Extended software (Adobe Systems Inc, San Jose, CA, USA) and wound contraction percentage was calculated using the following formula: Percentage of wound contraction = (A0 – At ) / A0 × 100
Where A0 is the original wound area and At is the wound area at the time of imaging. All rats were closely observed for any infection and if they showed signs of infection were separated, excluded from the study and replaced.
Histological studies
The tissue samples were taken on 7, 14, 21 days after surgery from periphery of the wound along with normal skin and fixed in 10% buffered formalin, dehydrated and embedded in paraffin wax, sectioned at 5 µm and stained with hematoxylin and eosin (H&E) stains. Photomicrographs were obtained under light microscope to assess the predominant stage of wound healing. Three parallel sections were obtained from each specimen. Cellular infiltration including the number of mononuclear cells, polymorphonuclear cells and fibroblastic aggregation were quantitatively evaluated.
Determination of hydroxyproline levels
On the day 21 after surgery, a piece of skin from the healed wound area was collected and analyzed for hydroxyproline content. As a major part of collagen, hydroxyproline has an essential role in collagen stability. The collagen is the major component of extracellular tissue, which gives support and strength. Tissues were dried in a hot air oven at 60–70 ◦C to constant weight and were hydrolyzed in 6N HCl at 130 ◦C for 4 h in sealed tubes. The hydrolysate was neutralized to pH 7.0 and was subjected to Chloramine-T oxidation for 20 min. The reaction was terminated by addition of 0.4M perchloric acid and color was developed with the help of Ehrlich reagent at 60 ºC and measured at 557 nm using UV-visible spectrophotometer.
Statistical analysis
Differences among groups were evaluated by Kruskal–Wallis variance analysis. When the P-value from the Kruskal–Wallis test statistics was statistically significant, multiple comparison tests were used to know differences. Student’s t-test was used for evaluation of test results. SPSS 11.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A P-value was set at 0·05.
Results
Microbiology
In animals of group IV whose infected wounds were treated with nano selenium, the counts of S. aureus cultured in the wound tissues were significantly lower than in the infected wounds in groups II and III (p=0.001). No animals died due to infection or anesthetics. The uninfected wounds had no CFU/g of S. aureus count. Local application of nano selenium in combination with chitosan biofilm significantly reduced the rate of total bacterial count on 7 and 14 days post-wounding compared in groups II and III (p=0.001).
Planimetric findings
Wound contraction percentage in different groups within the study period is shown in Figure 2. The healing rate of wounds in group IV was significantly different compared to groups II and III (p= 0.001).
Fig. 2. Line graph indicating reduction in wound area in experimental groups. Results were expressed as mean ± SEM. * P<0.05 vs. other experimental groups.

Histological findings
There were significant differences in comparisons of group IV and other groups, particularly in terms of cellular infiltration and neovascularization. During the study period, scores for neovascularization was significantly higher in group IV rats than other groups (p<0.05). Polymorphonuclear (PMN) and mononuclear (MNC) cell count and fibroblast cell proliferation in group IV were significantly higher than those of other experimental groups (p<0.05) (Table 1) (Figures 3-6).
Fig. 3. Box-and-whisker plots of number of polymorph nuclear cells in excisional model of the rat’s skin in experimental groups. Results were expressed as mean ± SEM.

Fig. 4. Line graph indicating number of momonuclar cells in excisional model of the rat’s skin in experimental groups. Results were expressed as mean ± SEM. * P < 0.05 vs other experimental groups.

Fig. 5. Box-and-whisker plots of number of fibroblasts in excisional model of the rat’s skin in experimental groups. Results were expressed as mean ± SEM.
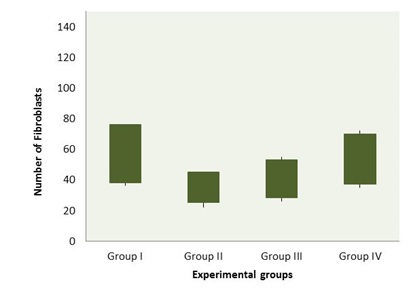
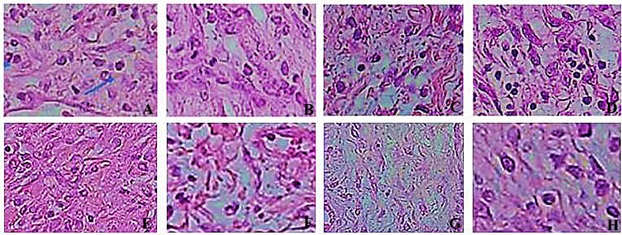
Fig. 6. Histological characteristics of rat skin on the days 7 (First row) and 14 (Second row) after wound creation in excisional wound model in experimental groups. A to D groups I to IV, respectively, and E to H groups I to IV. Wounds with surrounding skin were prepared for histological microscopic evaluation by H&E staining (×400).
Findings of hydroxyproline levels
Proline is hydroxylated to form hydroxyproline after protein synthesis. Hydroxyproline contents in groups I to V were found to be 49.62±2.54, 75.19±3.88, 77.12±2.73 and 97.12±3.57 mg/g, respectively. Hydroxyproline contents were significantly increased in the group IV which implies more collagen deposition compared to groups II and III (p=0.001).
Discussion
Although the wound healing process occurs by itself, spontaneously, and does not require much help, there are various risk factors such as infection, supply of blood, nutritional status and other factors that influence the resolution of this process [21]. It is well known that attack by microbes, which invade the skin barrier, delays the natural wound healing process [22]. MRSA is increasing in infections and is a serious threat to patients in health care facilities and the community. There are many reports in the literature that researchers have been working on various scaffolds and agents to combat MRSA related infections [23-33]. Resistance to common antibiotics makes treating MRSA costly and difficult. The main end point observed in this study, wound contraction and reduction in wound area, was accelerated by treating the wounds with chitosan and nano selenium. All the parameters observed (presence of necrotic tissue, clotting and crust, re-epithelialization and granulation tissue growth, bacterial count) were affected; suggesting that chitosan and nano selenium was effective against MRSA. Local application of chitosan and nano selenium biofilm at the wound site produced significant wound healing activity.
In excisional wound model there was a significant decrease in wound area. This indicated improved collagen maturation by increased cross linking. The balance between synthesis and breakdown and so deposition of collagen is important in wound healing and development of wound strength [34]. Hydroxyproline is a major component of the collagen that permits the sharp twisting of the collagen helix. It helps on providing stability to the triple-helical structure of collagen by forming hydrogen bonds. Hydroxyproline is found in few proteins other than collagen. For this reason, hydroxyproline content has been used as an indicator to determine collagen content [35]. Increase in hydroxyproline content in group V indicated increased collagen content, since hydroxyproline is the direct estimate of collagen synthesis.
Nanoparticles (NPs) have become significant in the regenerative medicine field in the last two decades [36]. Many biological processes happen at through mechanisms that fundamentally act at the nanometer scale. Thus, materials such as NPs can be used as unique tools for drug delivery, imaging, sensing, and probing biological processes [37]. In the context of wound healing, the special properties of NPs like electric conductivity, antimicrobial activity, and high surface to volume ratio, swelling, and contraction make NPs versatile resources.
Several reports have demonstrated that there is a beneficial effect of chitosan as a biologically active dressing in wound management. It has been reported that the application of chitosan to the open wounds in dogs induced exudate, which has a high growth factor activity, and induced infiltration by inflammatory cells and granulation tissue formation accompanied by angiogenesis [38,39]. Chitosan-membrane-based wound products have been investigated both in laboratory animals and humans, however, are still at the early stages of development. Since 1980, chitosan and its derivatives have been used in skin and wound management products in Japan. Beschitin W, an artificial skin prepared from chitin threads, has been developed for human use and is on the market [40,41].
We selected chitosan as a dressing material due to its biocompatibility, biodegradability, haemostatic activity, anti-inflectional activity and property to accelerate wound healing [42]. The N-acetyl glucosamine (NAG) present in chitin and chitosan is a major component of dermal tissue which is essential for repair of scar tissue. Its positive surface charge enables it to effectively support cell growth and promotes surface induced thrombosis and blood coagulation. Free amino groups which are present on the chitosan membrane surface may form polyelectrolyte complexes with acidic groups of the cellular elements of blood [42]. It has several advantages over other type of disinfectants because it possesses a higher antimicrobial activity, a broader spectrum of activity, a higher killing rate and a lower toxicity toward mammalian cells. However, synthetic polymers are available at a lower price than biopolymer chitosan, substitution of chitosan by these synthetic polymers could reduce the price of chitosan-based films with safe effect on their functionality [42]. The use of nanocomposites containing metals and metal oxides allows the therapeutic use of both unique properties of nanoparticles and polymer matrix properties. In this case, often the use of nanoparticles in nanocomposite structure allows not only to increase the stability of nanoparticles but also to reduce their potential cytotoxicity [43].
Selenium is a nonmetal that has some metal properties. The use of selenium in nanoform is promising for regenerative medicine. It is well known that nanoselenium is a highly effective long-acting antioxidant. Its local introduction in the area of injury can lead to violation of redox signaling. Selenium nanoparticles have been investigated for various medical applications and as a potential material for orthopedic implants [44]. Currently, studies which indicate precisely the ability of the selenium compounds to inhibit bacterial growth and formation of bacterial biofilms are also available [44]. Selenium has reported to show significant antiproliferative activity against HeLa and HepG2 cell lines [45]. The wound healing activity of selenium nanoparticles have revealed that 5% selenium ointment heals the excision wound of Wistar rats up to 85% within 18 days compared to the standard ointment [45].
Biomaterials derived from natural products can provide materials with greater complexity and composition. In order to mimic the extracellular matrix (ECM) conditions of the wound and to provide a scaffold for the fibroblasts for collagen deposition, ECM-based therapies have gained popularity [46]. A phase I clinical trial using fibroin to enhance wound healing is currently underway. Finally, there have been numerous marine polysaccharide hydrogels like marine collagen from Stomolophus nomurai meleagris, Oncorhynchus keta, Lates calcarifer, Stichopus japonicas, and Salmo salar, alginate from Macrocystis pyrifera, chitosan from crabs and shrimps, which are bioactive and increase wound healing rates in mice [25]. In the present study, Chitosan/Nano Selenium biofilm film revealed that there was a significant difference by means of histopathological examinations in group IV compared to other experimental groups and showed significant effect on inflammatory infiltration and number of fibroblasts in time-dependent activity. This showed promising effect of Chitosan/Nano Selenium biofilm on wound healing.
In conclusion,Chitosan/Nano Selenium biofilm resulted in significant improvement in planimetric and histopathological indices in full thickness wound healing. Thus, from this study it could be concluded that Chitosan/Nano Selenium biofilm have a reproducible wound healing potential and hereby justifies its use in practice.
Acknowledgments
This study was a part of thesis in partial fulfillment of M.Sc. degree in anatomical Sciences at Department of Anatomical Sciences, Faculty of Medicine, Lorestan University of Medical Sciences. Authors would like to acknowledge the Deputy Dean for Research for support of this study.
Conflict of interests: None
- Srinivas Reddy B, Kiran Kumar Reddy R, Naidu VG, Madhusudhana K, Agwane SB, Ramakrishna S, et al. Evaluation of antimicrobial, antioxidant and wound-healing potentials of Holoptelea integrifolia. J Ethnopharmacol. 2008;115(2):249-56.
- Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. 2015;5(7):509-14.
- Calfee DP. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, and other Gram-positives in healthcare. Curr Opin Infect Dis. 2012;25(4):385-94.
- Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, et al. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012;39(2):96-104.
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763-71.
- Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325(5944):1089-93.
- Braine T. Race against time to develop new antibiotics. Bull World Health Organ. 2011;89(2):88-9.
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36(6):697-705.
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106(1):256-61.
- Tran PA, Zhang L, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1097-114.
- Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274(1-2):1-33.
- No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74(1-2):65-72.
- Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34(12):1515-20.
- Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR, et al. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33-64
- Oyarzun-Ampuero F, Vidal A, Concha M, Morales J, Orellana S, Moreno-Villoslada I. Nanoparticles for the Treatment of Wounds. Curr Pharm Des. 2015;21(29):4329-41.
- Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42(10):1524-33
- Gao X, Zhang J, Zhang L. Hollow sphere selenium nanoparticles: their in‐vitro anti hydroxyl radical effect. Advanced Materials. 2002;14(4):290-3.
- Bai Y, Wang Y, Zhou Y, Li W, Zheng W. Modification and modulation of saccharides on elemental selenium nanoparticles in liquid phase. Materials letters. 2008;62(15):2311-4.
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chemistry. 2010;122(1):161-6.
- Kumar MS, Kirubanandan S, Sripriya R, Sehgal PK. Triphala promotes healing of infected full-thickness dermal wound. J Surg Res. 2008;144(1):94-101.
- Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Med Clin North Am. 2013;97(5):911-46.
- Pattanayak SP, Sunita P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J Ethnopharmacol. 2008;120(2):241-7.
- Jonaidi Jafari N, Kargozari M, Ranjbar R, Rostami H, Hamedi H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J Food Process Preserv. 2018;42(1):e13336.
- Maghsoudi O, Ranjbar R, Mirjalili SH, Fasihi-Ramandi M. Inhibitory Activities of Platelet-Rich and Platelet-Poor Plasma on the Growth of Pathogenic Bacteria. Iran J Pathol. 2017;12(1):79-87.
- Ranjbar R, Shahreza MHS, Rahimi E, Jonaidi-Jafari N. Methicillin-resistant Staphylococcus aureus isolates from Iranian restaurant food samples: Panton-Valentine Leukocidin, SCCmec phenotypes and antimicrobial resistance. Trop J Pharm Res. 2017;16(8):1939-49.
- Mohammadi R, Mehrtash M, Mehrtash M, Hassani N, Hassanpour A. Effect of Platelet Rich Plasma Combined with Chitosan Biodegradable Film on Full-Thickness Wound Healing in Rat Model. Bull Emerg Trauma. 2016;4(1):29-37.
- Memariani H, Shahbazzadeh D, Ranjbar R, Behdani M, Memariani M, Pooshang Bagheri K. Design and characterization of short hybrid antimicrobial peptides from pEM-2, mastoparan-VT1, and mastoparan-B. Chem Biol Drug Des. 2017;89(3):327-338.
- Ranjbar R, Goudarzi MM, Jonaidi N, Moeini R. Cassette chromosome mec typing of methicillin-resistant Staphylococcus aureus isolates from patients in Tehran. Molecular Genetics, Microbiology and Virology. 2016;31(2):109-15.
- Ranjbar R, Goudarzi MM, Jonaidi N. Prevalence of mecA and femB genes in methicillin-resistant Staphylococcus aureus isolated from Iran's military hospitals. Journal of Pure and Applied Microbiology. 2016;10(1):389-94.
- Rostami H, Mohammadi R, Asri-Rezaei S, Tehrani AA. Evaluation of Application of Chitosan/nano Sodium Selenite Biodegradable Film on Full Thickness Excisional Wound Healing in Rats. Iran J Vet Surg. 2018;13(1):14-22.
- Kazemi-Darabadi S, Akbari G, Jarolmasjed S-H, Shahbazfar A-A. A Histopathologic study of effects of olive oil plus lime water on third-degree burn in mouse model. Iran J Vet Surg. 2017;12(1):55-63.
- Javanmardi S, Divband B. Beneficial Effects of Ag-Exchanged Zeolite Nanocomposite on Excisional Wound in Rats. Iran J Vet Surg. 2017;12(1):25-32.
- Shabrandi A, Azizi S, Hobbenaghi R, Ownagh A, Keshipour S. The healing effect of chitosan supported nano-CeO2 on experimental excisional wound infected with pseudomonas aeruginosa in rat. Iran J Vet Surg. 2017;12(2):9-20.
- Dogan S, Demirer S, Kepenekci I, Erkek B, Kiziltay A, Hasirci N, et al. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int Wound J. 2009;6(2):107-15.
- Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol. 2010;130(1):38-48.
- Mclaughlin S, Podrebarac J, Ruel M, Suuronen EJ, McNeill B, Alarcon EI. Nano-engineered biomaterials for tissue regeneration: what has been achieved so far? Front Mater. 2016;3:27.
- Wang EC, Wang AZ. Nanoparticles and their applications in cell and molecular biology. Integr Biol (Camb). 2014;6(1):9-26.
- Okamoto Y, Shibazaki K, Minami S, Matsuhashi A, Tanioka S, Shigemasa Y. Evaluation of chitin and chitosan on open would healing in dogs. J Vet Med Sci. 1995;57(5):851-4.
- Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, et al. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A. 2003;64(1):177-81.
- Kifune K. Clinical application of chitin artificial skin (Beschitin W). Advances in chitin and chitosan; 1992. p. 9-15.
- Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69(2):216-22.
- Archana D, Dutta J, Dutta PK. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol. 2013;57:193-203.
- Li X, Wang H, Rong H, Li W, Luo Y, Tian K, et al. Effect of composite SiO₂@AuNPs on wound healing: in vitro and vivo studies. J Colloid Interface Sci. 2015;445:312-319.
- Wang Q, Webster TJ. Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J Biomed Mater Res A. 2012;100(12):3205-10.
- Ramya S, Shanmugasundaram T, Balagurunathan R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol. 2015;32:30-9.
- Das S, Baker AB. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng Biotechnol. 2016;4:82.

